Solar energy for dummies part 3
For those of you who liked the previous solar posts, here comes the last part. I will go deeper into the matter of photovoltaic systems (being a complete beginner in this field myself). Let’s start with a short repetition of the last blog entries:
A solar module consists of several solar cells that convert sunshine directly into electric energy. There are rigid and flexible modules and on-grid and off-grid photovoltaic systems. What matters most in a solar cell is the crystal structure: the brick solar module consists of monocrystalline cells, furthermore there are polycrystalline and amorphous cells. The degree of efficiency of solar cells decreases in the course of the time which is due to degradation or pollution of the glasses. But even if it does not function anymore one time, it is still recyclable to up to 95%.
But now we want to learn something new: we are going to have a close look into the solar cell. Solar cells are electronic components with which solar energy is directly transformed into electrical energy. We already understood that. But what exactly do solar cells consist of? Most solar cells are made of silicon which is a semiconductor material that is gained from ordinary sand. In contrast to an isolator, a semiconductor is a material that possesses several free electrons under normal environmental conditions (room temperature). This is why current can flow in a semiconductor which is not possible in an isolator – and this is also where the name derives from: SEMIconductor. The current is much lower than in an electrical conductor (wire, for example). In a nutshell: 1. An electrical conductor conducts current (for example wire, water, coal, saline solution, silver, gold), a semiconductor partly conducts current (silicon, for example) and an isolator does not conduct current at all (for example rubber, glass, ceramic, amber, oil).
With the help of some technical tricks, engineers are able to specifically increase semiconductors’ conductivity. They simply build chemical elements into the silicon crystal which either leads to more free electrons: this surplus of electrons is called “n type”. Or they build in chemical elements that generate a lack of electrons in the silicon: this lack of electrons leads to several free places in the silicon crystal that are not occupied. In this case, we speak of holes. These holes, as well as electrons, are moving freely. This surplus of holes is called “p type” (positively conducting).
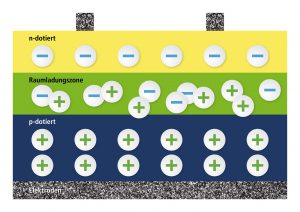
When producing solar cells, there will be a layer with a surplus of electrons above a layer with a lack of electrons in the silicon crystal. This leads to the freely moving electrons determining in the holes in a thin transition layer (plus and minus attracting each other, as with magnets). A thin layer, called space-charge region, develops that does not include neither free electrons nor free holes – but that is an isolating layer, so to say. If we create a contact at the n-layer and at the p-layer and connect it, there won’t happen anything as both are isolated from each other. An isolator, so we’ve learned, does not conduct current.
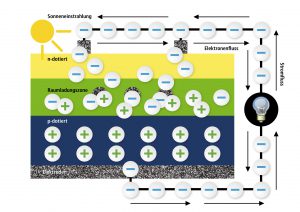
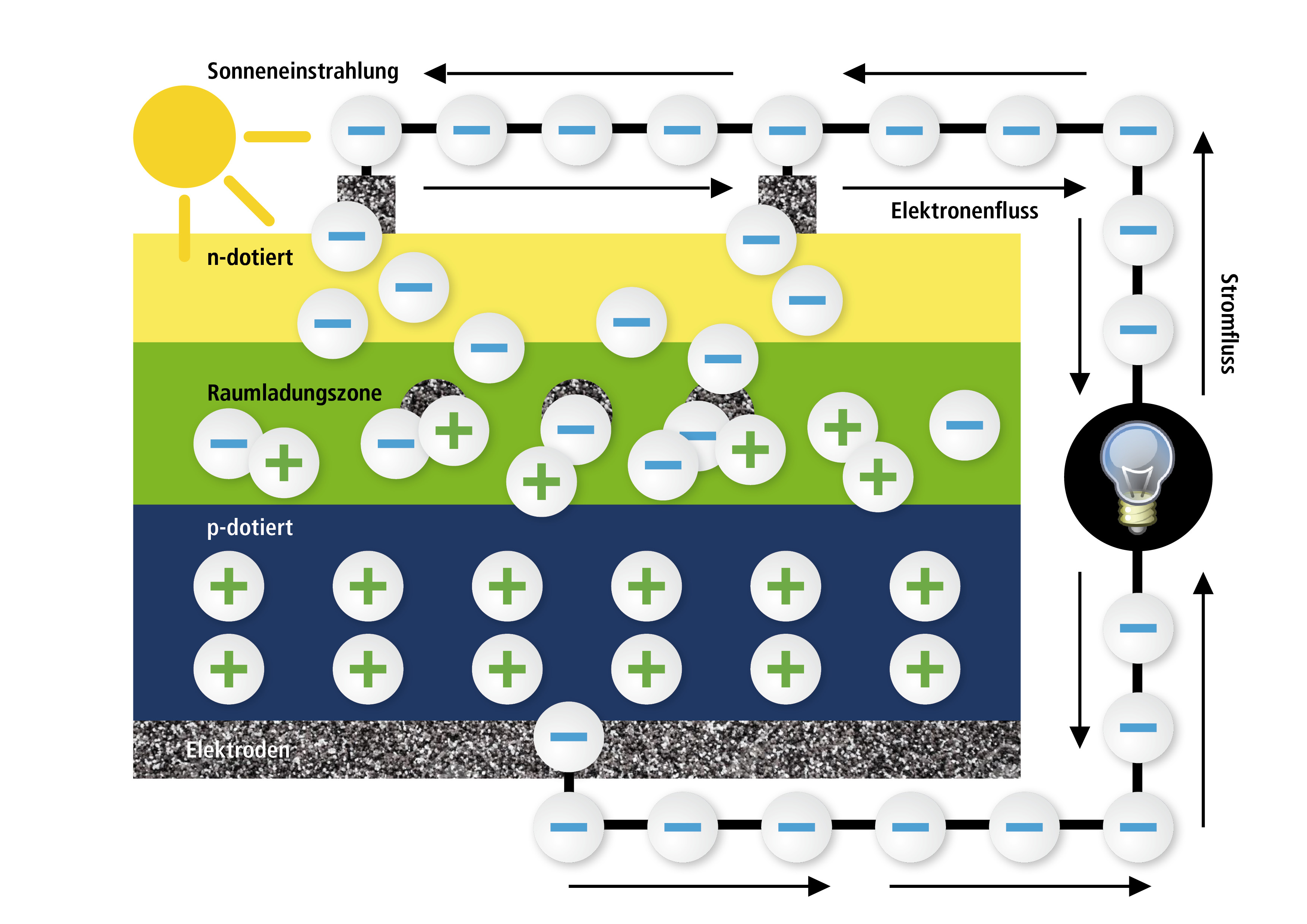
We will be dealing with the sun now! Sunrays as we see them consist of particles, so called photons – a fact that the famous Albert Einstein once realized. The sunrays meet the solar cell’s surface and penetrate the thin n-layer until they reach the transition layer where they can knock out the electrons that are connected to the holes. This can be compared to playing with marbles when you shoot one marble with your own one out of the way. The free electrons now flow back to the free holes via the connected electrodes which leads to a current flow outside of the solar cell. This current is the solar current that we were looking for. With it, you can drive engines, lighten up lamps or charge accumulators.
Pretty complicated! Now we understand what happens exactly in a solar cell.

 en
en de
de es
es